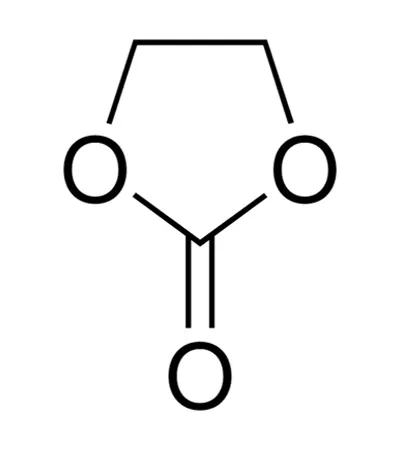
Ethylene Carbonate (EC) is a high permittivity organic solvent with remarkable dielectric properties of great appeal in the electrolytes of lithium-ion batteries. Having the reputation of being able to grow a robust Solid Electrolyte Interphase (SEI) over graphite anodes, EC becomes critical in making batteries safer, more efficient, and with a longer life. As a co-solvent in electrolyte preparation, it facilitates optimal lithium-ion flow and increases cell stability.
This tranquility is the ultimate value in thermally stable and safe energy storage for Ethylene Carbonate. It is often combined with low viscous solvents, such as Dimethyl Carbonate (DMC), which offer an excellent bond between ionic conductivity and viscosity. Under the rapid growth of demand for solutions to store energy in electric vehicles, consumer electronics, and renewable energy grids, EC has remained a key component involved in the delivery of reliable and long-lasting power storage systems that are high-performing.